Blog by: Shelly Maffia, MSN, MBA, RN, LNHA, QCP, CHC, CLNC, CPC-A, Director of Regulatory Services, Proactive Medical Review and Jessica Cairns, RN, RAC-CT, CMAC, Clinical Consultant, Proactive Medical Review
July 29th, 2021, the Skilled Nursing Facility (SNF) prospective payment system (PPS) final rule was released. The rule, which goes into effect October 1, 2021, contained several updates, including factors affecting the usual payment rates, changes to diagnosis code mapping under the Patient Driven Payment Model (PDPM), and updates to both the SNF Quality Reporting Program (QRP) and SNF Value-Based Purchasing (VBP) Program. In addition, there was discussion surrounding the much-debated future PDPM parity adjustment which considers how SNFs will pay back the estimated $1.7 Billion “overpayment” for the first year of PDPM. In this blog, we will take a look at some of the biggest takeaways affecting our business and how to prepare.
Medicare Part A Rates
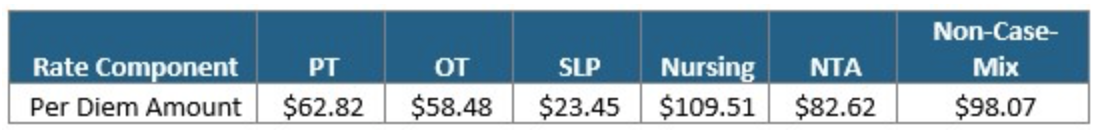
The Federal Per Diem rates are updated annually and take effect every October 1st. The typical “raise” SNFs receive is over 2%. This October, CMS anticipates a 1.2% rate increase, which equates to approximately $411 million more in PPS reimbursement as compared to 2021. This is based on an unadjusted increase of 2.7% reduced by both a 0.08% forecast error and a 0.07% productivity adjustment. The unadjusted per diem components of the rates for FY 2022 are listed below for both urban and rural providers. Of these rates, 70.4% of each component is adjusted by the wage index, which varies for each core-based statistical area. Listed below are the unadjusted rates for October 1st, 2021.
Unadjusted Federal Rate Per Diem-Urban
Unadjusted Federal Rate Per Diem-Rural
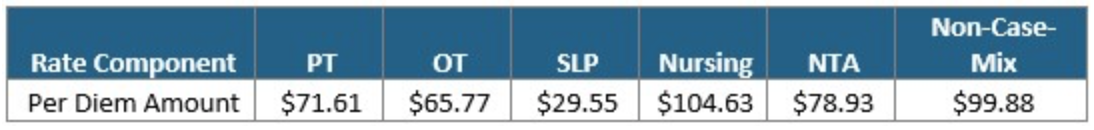
To give you an idea of the daily rate changes [urban] from FY2021 to FY2022, the PT component will increase $0.78/day, OT component to $0.73/day, SLP to $0.29/day, Nursing to $1.35/day, NTA to $1.02/day and the Flat Rate $1.22/day.
Delayed PDPM Parity Adjustment
SNF’s can celebrate this small victory. The parity adjustment was the top concession that CMS made in response to feedback on the proposed rule. This proposed rule left us with the potential of $1.7 billion (5%) parity reduction as CMS data supported that PDPM was not budget neutral as it intended. Said differently, depending on the different component combinations, the rate could have been $10-48.00 per day lower. While we get a pass this year, the rate recalibration will be re-examined in the Proposed Rule for FY2023.
ICD-10-CM code mappings for PDPM classification
The final rule contained updates to the mapping of several diagnoses and where they are classified under the PDPM. Some of the conditions affected include the following:
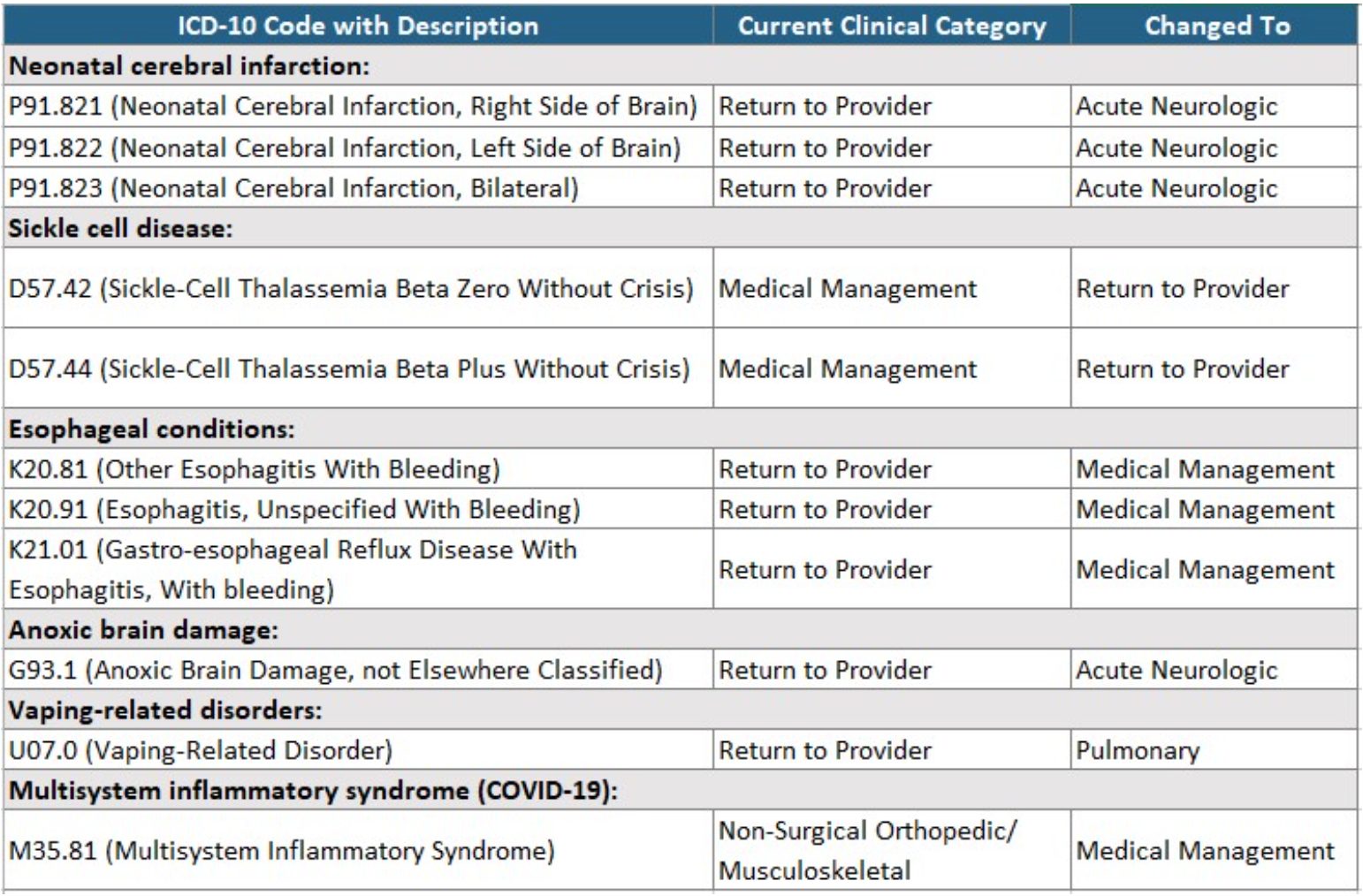
The FY 2022 PDPM ICD-10 Mapping file is available at https://www.cms.gov/files/zip/fy-2022-pdpm-icd-10-mappings.zip
HIV Add-On
The add-on for HIV was renewed and remains unchanged from prior years, including a 12.8 percent increase to the nursing component and an additional add-on of 8 points to the non-therapy ancillary (NTA) component. This add-on is based on claims data containing a diagnosis code for HIV or AIDS (B20).
VBP Program
CMS is suppressing the use of SNF readmission measure data for purposes of scoring and payment adjustments in the FY 2022 SNF VBP Program Year as a result of the PHE. They will use the previously finalized performance period (4/1/2019-12/31/2019 and 7/01/2020-09/30/2020) and baseline period (FY 2019) to calculate each SNF’s RSRR for the SNFRM and assign all SNFs a performance score of zero in the FY 2022 SNF VBP Program Year, resulting in all SNFs receiving an identical performance score and incentive payment multiplier. SNFs will not be ranked for the FY 2022 SNF VBP program.
CMS will reduce each participating SNF’s adjusted Federal per diem rate for FY 2022 by 2 percentage points and award each participating SNF 60 percent of that 2 percent withholding, resulting in a 1.2 percent payback for the FY 2022 SNF VBP Program Year. Those SNFs subject to the Low-Volume Adjustment policy (fewer than 25 eligible stays during the performance period) would receive 100 percent of their 2 percent withhold.
For FY2024, the performance period will be FY 2022 and the baseline period will be FY2019.
Currently, the SNF VBP program only includes the readmission measure. CMS is considering adding additional measures in the future. The table below shows the additional measures under consideration, in addition to these measures, CMS is also considering adding a measure related to staff turnover.
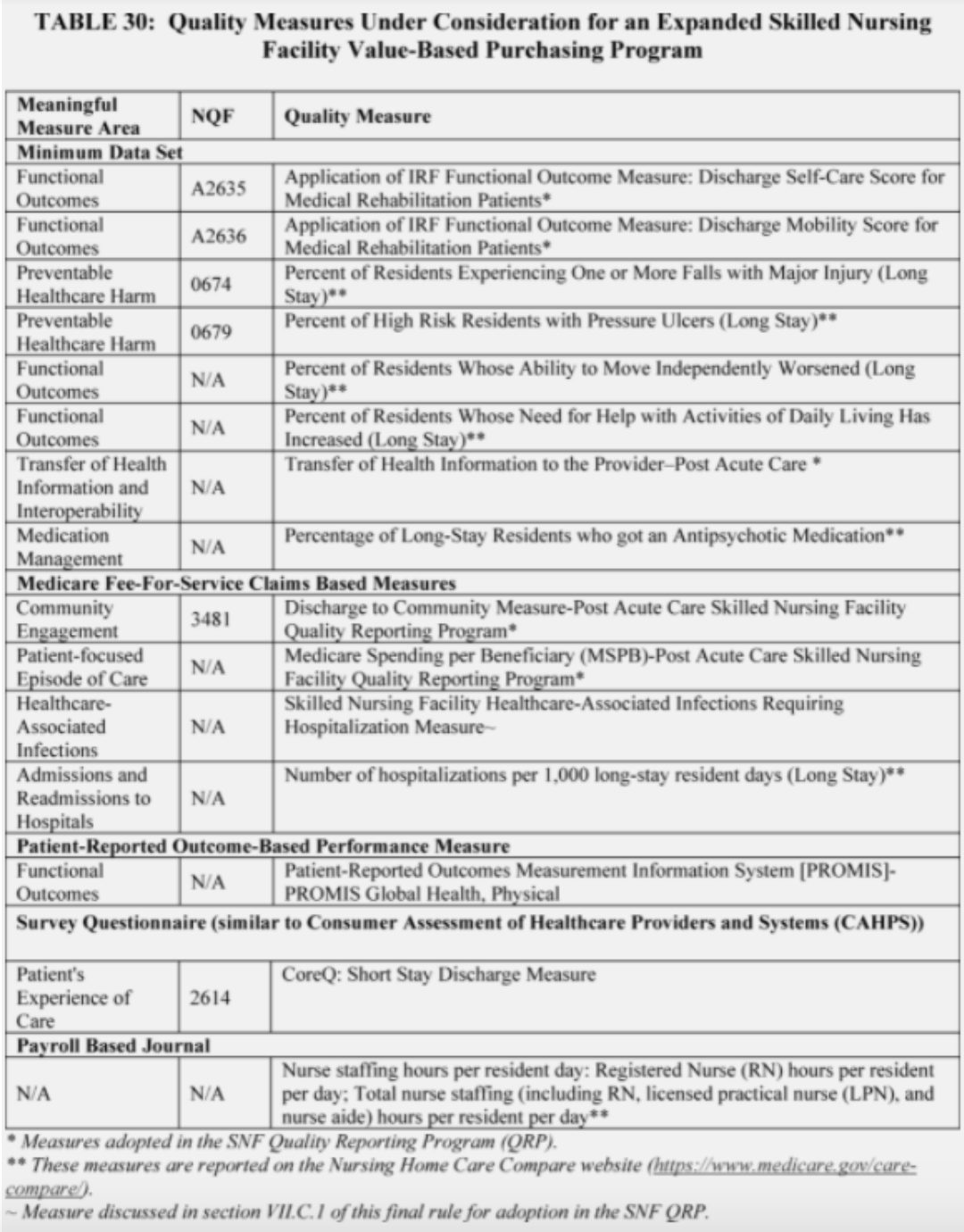
(SOURCE: Federal Register)
Consolidated Billing
Effective with items and services furnished after 10/01/2021, CMS has established an additional category of excluded codes for certain blood clotting factors for the treatment of patients with hemophilia and other blood clotting disorders, which includes those identified by HCPCS codes J7170, J7175, J7177-J7183, J7185-J7190, J7192-J7195, J7198-J7203, J7205, and J7207-J7211. The latest list of excluded codes can be found on the SNF Consolidated Billing website at https://www.cms.gov/Medicare/Billing/SNFConsolidatedBilling.
QRP
The SNF QRP currently has 13 measures for the FY 2022 SNF QRP:
- MDS Assessment-Based
- Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury
- Application of Percent of Residents Experiencing One or More Fall with Major Injury (Long Stay) (NQF #0674)
- Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (NQF #2631)
- Application of IRF Functional Outcome Measure: Change in Mobility Score for Medical Rehabilitation Patients (NQF#2634)
- Application of IRF Functional Outcome Measure: Discharge Mobility Score for Medical Rehabilitation Patients (NQF#2636)
- Application of IRF Functional Outcome Measure: Change in Self-Care Score for Medical Rehabilitation Patients (NQF#2633)
- Application of IRF Functional Outcome Measure: Discharge Self-Care Score for Medical Rehabilitation Patients (NQF#2635)
- Drug Regimen Review Conducted with Follow-Up for Identified Issues- Post Acute Care (PAC) Skilled Nursing Facility (SNF) Quality Reporting Program (QRP)
- Transfer of Health Information to the Provider Post-Acute Care
- Transfer of Health Information to the Patient Post-Acute Care
- Claims-Based
- Medicare Spending Per Beneficiary (MSPB) – PAC SNF QRP
- Discharge to Community – PAC SNF QRP (NQF #3481)
- Potentially Preventable 30-day Post-Discharge Readmission Measure for SNF QRP
CMS will adopt two new SNF QRP measures beginning with the FY 2023 SNF QRP:
- SNF Healthcare-Associated Infections Requiring Hospitalization measure –
- Will use FY 2019 claims data to calculate this measure for the FY 2023 QRP.
- This measure will be publicly reported beginning with the April 2022 Care Compare refresh.
- COVID-19 Vaccination Coverage among Healthcare Personnel measure –
- Will use data submitted to NHSN by SNFs to calculate this measure with an initial data submission period from 10/1/2021-12/31/2021.
- Starting in CY 2022, SNFs will be required to submit data for the entire calendar year beginning with the FY2024 SNF QRP.
- This measure will be publicly reported beginning with the October 2022 Care Compare refresh or as soon as technically feasible using data collected for Q4 2021 and the most recent quarter of data will be reported during each advancing Care Compare refresh.
In addition, CMS is also updating the denominator for the Transfer of Health Information to the Patient PAC measure to exclude residents discharged home under the care of home health or hospice service.
How to Prepare
Make plans to share this information and assess the impact on your facility over the next two months in preparation for the October 1 effective date.
Quick list of action items:
- Review the rate changes including modifications to VBP adjustments, to determine the financial impact they will have on your organization
- Incorporate updated rates into your budget and plan accordingly
- Ensure the billing office is up to date on the current components that affect Medicare rates. This includes ensuring updates to billing software.
- Discuss the changes in the ICD-10 mapping with the appropriate staff and include the new consolidated billing exclusions related to blood-clotting factors in that conversation.
- Provide education to clinical staff on changes to VBP and QRP and verify you have processes in place to report all required information.
- Ensure the infection control nurse has a process in place to report required vaccine information to the CDC.
- Continue to monitor facility readmission rates and ensure a process is in place to mitigate unnecessary rehospitalizations.
References
Click here to continue reading this blog.
About Proactive Medical Review
HTS partners with Proactive Medical Review, a third party company who specializes in ensuring compliance with regulatory standards and promoting measurable care excellence. The team includes SNF experienced nurse, MDS, Health Facility Administrator, therapist and reimbursement specialists with experience serving in multi-site contract therapy operations, as corporate directors of quality, clinical program specialists, and Compliance Officers. Proactive is uniquely positioned to assist in managing the many changes and challenges facing providers partnered with HTS. Learn more about our commitment to compliance here.




